
It can also be obtained through supplements, and it is important to ensure that the body is getting enough calcium to maintain optimal health. Before emission Number i VBe After emission Units. How can I find the molar mass of an element The molar mass of elements is found by looking at the atomic mass of the element on the periodic table. It is also sometimes called: Molecular Mass, Molecular Weight, Formula Mass, or Formula Weight. Assuming that the isotope is stationary to begin with, find the wavelength of the y ray. One atomic mass unit (u) is equal to 1/12 the mass of one atom of carbon-12. An atomic mass unit is equal to 1/12 the mass of a single atom of carbon-12, the most abundant isotope of carbon, or 1.660538921 × 10 24 gram. An isotope of beryllium (atomic mass of 7.017 u) emits ay ray and recoils with a speed of 2.07 x 104 m/s.
#ATOMIC MASS FREE#
1 Da is defined as 112 of the mass of a free carbon-12 atom at rest in its ground state. Although the SI unit of mass is the kilogram (symbol: kg), atomic mass is often expressed in the non-SI unit dalton (symbol: Da) equivalently, unified atomic mass unit (u). Calcium is found in a variety of foods, including dairy products, leafy green vegetables, nuts, and grains. atomic mass unit (AMU), also called dalton, in physics and chemistry, a unit for expressing masses of atoms, molecules, or subatomic particles. The atomic mass (ma or m) is the mass of an atom. It is necessary for the development and maintenance of strong bones and teeth, and it is also important for muscle function, nerve function, and blood clotting. Somma fra loro il numero di protoni e neutroni. For calcium, the atomic mass is 40.078 amu and the atomic number is 20, so the number of neutrons in the nucleus of a calcium atom is 40.078 - 20 = 20.078.Ĭalcium is an important element that is essential for the proper functioning of the human body. The atomic mass of an element is equal to the sum of the masses of its protons and neutrons, and the number of neutrons in the nucleus of an atom of an element can be determined by subtracting its atomic number from its atomic mass.
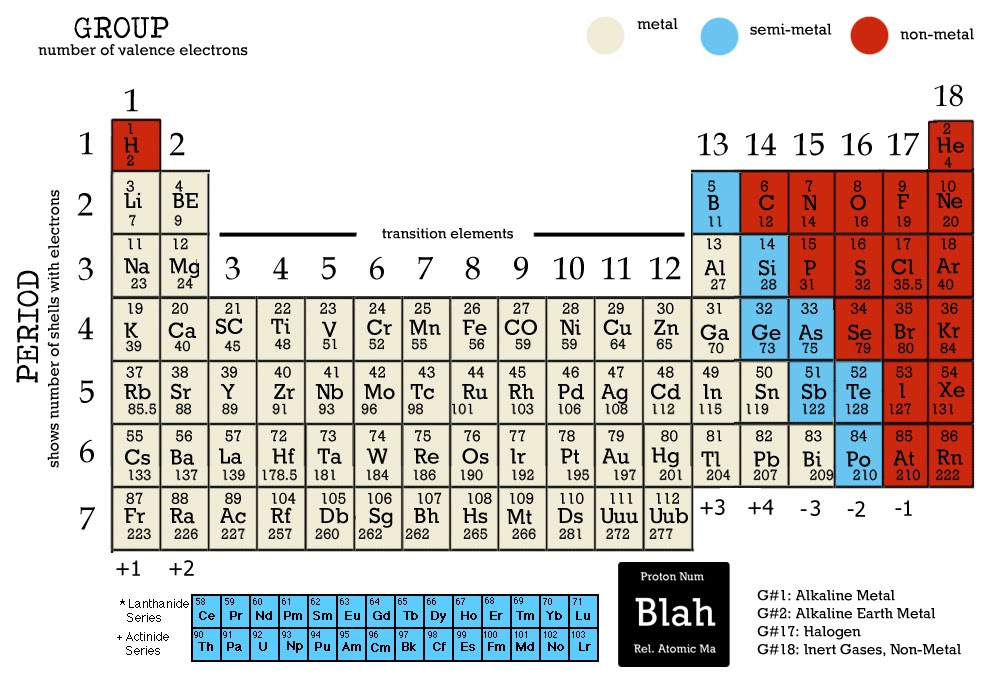

Calcium is a chemical element with the atomic number 20, which means that it has 20 protons in the nucleus of its atoms. The atomic mass of calcium (Ca) is 40.078 amu. The atomic mass of an element can be found on the periodic table, which is a chart that organizes elements according to their atomic number, which is the number of protons in the nucleus of an atom of that element. This is a list of chemical elements, sorted by atomic mass (or most stable isotope) and color coded according to type of element. The atomic mass of an element is determined by the number of protons and neutrons in the nucleus of its atoms. The atomic mass of an element is a measure of the mass of a single atom of that element, and it is typically expressed in atomic mass units (amu).


 0 kommentar(er)
0 kommentar(er)
